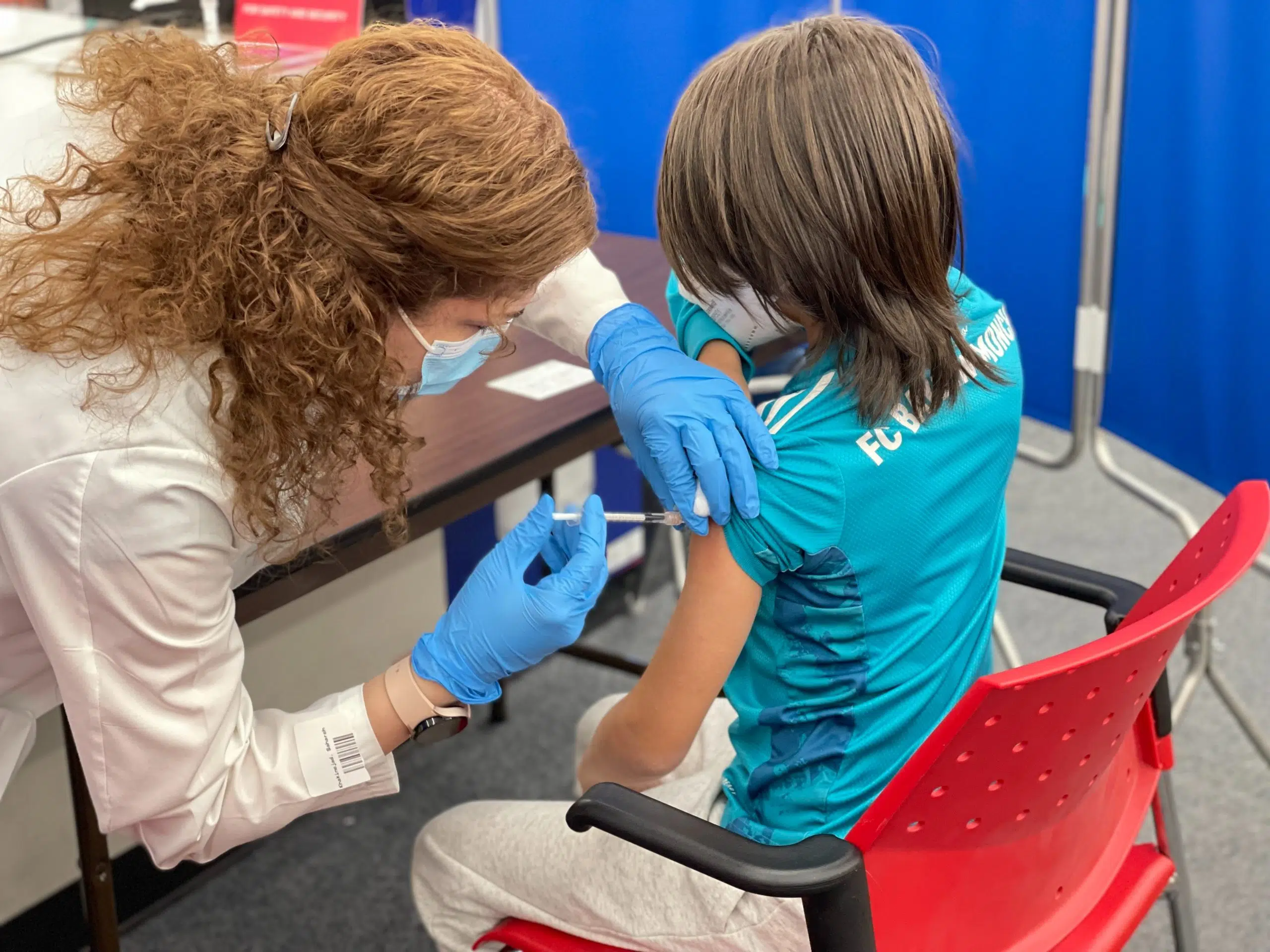
A new age group is likely to be eligible for the Covid-19 vaccine in the United States this week.
Children aged five and under might be eligible for a pandemic related vaccine from Pfizer BioNTech if it is approved by the U.S. Food and Drug Administration (FDA).
There have been studies conducted over the past two plus years of the pandemic on children in this age group with results showing positive outcomes and immunity rates.
The FDA is will be asking a separate group of vaccine experts to vet the studies and provide their recommendations. The Administration will then take that into account when they go to vote on approving the vaccine or not.
According to the Pfizer the vaccine, once approved, would be available in three doses to children from six months old up to five years old.
The vaccine was studied in the young age group on 10 symptomatic cases during the peak of the Omicron variant. Those cases showed an 80 per cent efficacy rate during the study, however that could also change as the study continues with the vaccine in the vulnerable under five group.
In Canada and the U.S., there are currently no vaccines approved for use in children under the age of five.
More on Pfizer BioNTech vaccine for kids under five, can be read here.